
Lumefantrine IP/USP
2000 INR/Kilograms
Product Details:
- HS Code 29339900
- Shelf Life 5 years
- Heavy Metal (%) Not more than 0.001%
- EINECS No 617-410-1
- Color Yellow
- Solubility Practically insoluble in water; soluble in methanol, ethanol, and dichloromethane
- Structural Formula Available on request
- Click to view more
X
Lumefantrine IP/USP Price And Quantity
- 2000 INR/Kilograms
- 5 Kilograms
- 2000.00 - 3000.00 INR/Kilograms
- Complies with IP/USP specifications
- Complies with ICH guidelines
- GMP certified facility
- Not applicable (racemic mixture)
- By IR, HPLC, and melting point
- Complies with pharmacopoeial limits
- Available with each batch
- HDPE drums with double polyliner
- Below 0.5%
- 25 kg / 50 kg
Lumefantrine IP/USP Product Specifications
- D90 < 15 microns
- For manufacturing of antimalarial drugs (Artemether-Lumefantrine combination)
- Bitter
- IP/USP
- Store in a cool, dry, well-ventilated place; away from direct sunlight
- C30H32Cl3NO
- Non-poisonous under prescribed dosage
- Not more than 0.5%
- 128-132C
- 528.94 g/mol
- Not more than 0.001%
- Solid
- 617-410-1
- Lumefantrine
- 82186-77-4
- 29339900
- Antimalarial Active Pharmaceutical Ingredient
- 5 years
- Practically insoluble in water; soluble in methanol, ethanol, and dichloromethane
- Yellow
- Odorless
- Available on request
- 98.0% minimum
- (RS)-2-(dibutylamino)-1-[(9Z)-2,7-dichloro-9-(4-chlorobenzylidene)-9H-fluoren-4-yl]ethanol
- Yellow crystalline powder
- Complies with IP/USP specifications
- Complies with ICH guidelines
- GMP certified facility
- Not applicable (racemic mixture)
- By IR, HPLC, and melting point
- Complies with pharmacopoeial limits
- Available with each batch
- HDPE drums with double polyliner
- Below 0.5%
- 25 kg / 50 kg
Lumefantrine IP/USP Trade Information
- ex our works
- Telegraphic Transfer (T/T), Letter of Credit (L/C), Cash in Advance (CID)
- 500 Kilograms Per Month
- 7 Days
- Contact us for information regarding our sample policy
- Standard export packing
- Middle East, Africa, Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe
- , Daman and Diu, Goa, Jharkhand, Odisha, Assam, Delhi, West India, Meghalaya, All India, South India, Central India, North India, Gujarat, Karnataka, East India, Kerala, Andhra Pradesh, Punjab, Dadra and Nagar Haveli, Arunachal Pradesh, Lakshadweep, Mizoram, Manipur, Bihar, Chandigarh, Chhattisgarh, Haryana, Himachal Pradesh, Maharashtra, Telangana, Madhya Pradesh, Tamil Nadu, Uttarakhand, Andaman and Nicobar Islands, Jammu and Kashmir, Nagaland, Tripura, Pondicherry, Rajasthan, Sikkim, Uttar Pradesh, West Bengal
- GMP and ISO certified Plant
Product Description
Lumefantrine Powder is used for the treatment of malaria and is generally prescribed in combination with artemether. It has chemical formula C30H32Cl3NO with molar mass of 528.939 g/mol. This compound is ideal for treating infections caused by Plasmodium falciparum and exerts its action against the erythrocytic stage. Lumefantrine Powder is known to be highly effective for infections acquired in chloroquine resistant areas. It is administered orally and has the ability to donate 1 and accept 2 hydrogen bonds.
Features:
-
It is known to have blood schizonticidal activity
-
Belongs to the group of arylamine alcohols
-
Has elimination half life of 4 to 6 days
High-Quality Manufacturing and Compliance
Our Lumefantrine is produced in India within a GMP-certified facility, ensuring adherence to global quality and safety standards. Each lot undergoes comprehensive testing, confirming compliance with IP and USP requirements, low impurity levels, and pharmacopoeial limits for residual solvents and microbial presence. This thorough process guarantees a reliable, high-quality API for antimalarial drug manufacturers.
Optimal Packaging and Stability
Lumefantrine is securely packed in HDPE drums equipped with a double polyliner, available in 25 kg and 50 kg sizes. This durable packaging safeguards the product against contamination and environmental factors, maintaining its efficacy and stability. The product should be stored in a cool, dry, well-ventilated area away from direct sunlight to preserve its 5-year shelf life.
Benefits for Pharmaceutical Development
Lumefantrines proven performance in combination therapies, notably with Artemether, makes it the preferred API in contemporary antimalarial drug manufacturing. Its outstanding purity, low impurity and heavy metal content, and strict compliance with international standards contribute to safer and more effective pharmaceutical preparations.
FAQs of Lumefantrine IP/USP:
Q: How is the quality of Lumefantrine ensured during manufacturing?
A: Lumefantrine is manufactured in a GMP-certified facility, following strict quality protocols. Each batch undergoes testing for identity (IR, HPLC, and melting point), impurity levels, microbial load, and residual solvents to fulfill IP/USP requirements. A Certificate of Analysis is provided with every shipment.Q: What is the recommended storage condition for Lumefantrine to maximize shelf life?
A: To maintain stability, Lumefantrine should be stored in a cool, dry, and well-ventilated location, protected from direct sunlight. Proper storage preserves its quality and ensures a shelf life of up to five years.Q: When is Lumefantrine used in pharmaceutical manufacturing?
A: Lumefantrine serves as the active pharmaceutical ingredient in antimalarial drug formulations, particularly in combination with Artemether. It is used during the drug manufacturing phase to produce effective therapies against malaria.Q: Where is Lumefantrine manufactured and supplied from?
A: Lumefantrine is manufactured in India and is available for export and import globally. It is supplied by reputed manufacturers, exporters, and suppliers, meeting international regulatory standards.Q: What benefits does Lumefantrine offer to pharmaceutical companies?
A: Pharmaceutical firms benefit from Lumefantrines high purity (98.0%), low impurity and heavy metal content, reliable pharmaceutical compliance, and extended shelf life, ensuring consistency, safety, and efficacy in antimalarial drug products.Q: What is the appearance and solubility profile of Lumefantrine?
A: Lumefantrine appears as a yellow crystalline powder and is practically insoluble in water, but dissolves well in methanol, ethanol, and dichloromethane. This solubility profile supports its application in pharmaceutical formulations.Q: How is Lumefantrine packaged for shipment and handling?
A: The API is packed in robust HDPE drums with a double polyliner for maximum protection. Standard packing sizes are 25 kg and 50 kg, ensuring the material stays uncontaminated and stable during transit and storage.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email

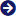




 Call Me Free
Call Me Free

